Blog

Advancing Alzheimer’s Management: Highlights from Our Scientific Session in Malta
In July, we hosted an insightful scientific session on Dementia in the beautiful Malta. The event gathered leading neurologists and neurosurgeons from North Macedonia.
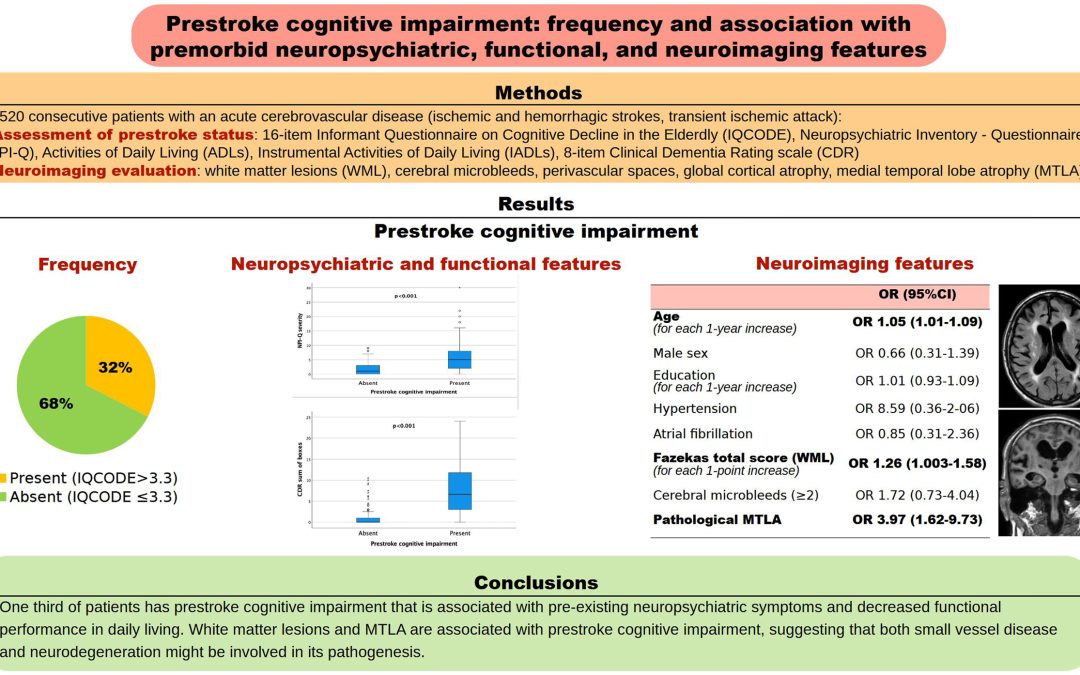
Understanding Prestroke Cognitive Impairment: Key Findings from Mele et al.’s Study
STROKE CLINICAL STUDY HIGHLIGHTS – Prestroke Cognitive Impairment: Frequency and Association With Premorbid Neuropsychiatric, Functional, and Neuroimaging Features by Mele F, Cova I, Nicotra A, et al.

Unlocking Potential: Webinar on Post-Stroke Recovery Therapies with Asian Stroke Advisory Panel
Moleac is delighted to support the upcoming webinar on Pharmacological Therapies for Post-Stroke Recovery hosted by the Asian Stroke Advisory Panel (ASAP) gathering neurology experts from Asia.
